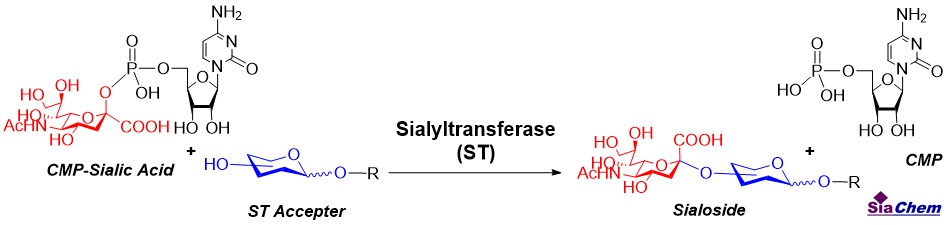

In eukaryotic cells, Neu5Ac is synthesized in the cytosol and then is transferred to nucleus and activated by cytosine 5′-monophosphate N-acetylneuraminic acid (CMP-Neu5Ac) synthetase to form CMP-Neu5Ac that then goes to Golgi to be transferred to glycoconjugates by sialyltransferases, which are subsequently secreted or delivered to cell surface. So far, twenty sialyltransferases have been identified for catalyzing the addition of sialic acids to terminal non-reducing position of the oligosaccharides of different sugar acceptors in different linkages on proteins and lipids [1,2]. Sialyltransferases normally locate at the Golgi apparatus as integral membrane proteins adding sialic acids to glycoconjugates during their syntheses. In addition, some sialyltransferases are also expressed as soluble enzymes [3] and sialyltransferases activity at plasma membrane are also reported in immune cells [4]. Each sialyltransferase presents high selectivity toward its acceptor substrate and create α2,3-, α2,6-, α2,8-linkages, respectively. Overall, the levels and linkages of sialic acids named as sialylation status are controlled by the levels and activities of sialyltransferases, which vary upon cell activation related to both physiological and pathological processes [5].