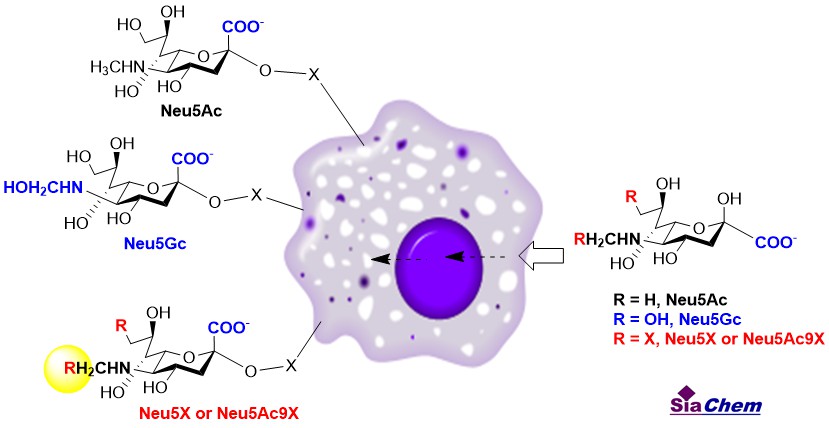

Sialic acids (Sias) are a family of 9-carbon containing acidic monosaccharides and often terminate cell surface glycans of either glycoproteins or glycolipids [1]. Sias are critical components of glycoconjugates that serve as receptors involved in many biological processes [2]. Metabolic sialo-engineering has been developed for modifying cell surface Sia with non-natural Sias [3]. This provides a very useful tool to investigate the biological function of Sia as part of sialoglycoconjugates in living cells. ]. Metabolic sialo-engineering can be achieved through the administration of Sia precursor analogues N-modified D-mannosamines, which are taken up, metabolized, and incorporated into cell surface sialoglycoconjugates of mammalian cells. In addition, Sias carrying modifications in the C-5 position (or C-9) can also be metabolically incorporated onto cell surface sialoglycoconjugates. One key advantage of using Sia analogues over Sia precursor analogues for the metabolic engineering of cell surface Sias is their independence from the multistep biosynthetic pathway. A range of non-natural Sia analogs has been successfully installed in a variety of cells and organisms [4].