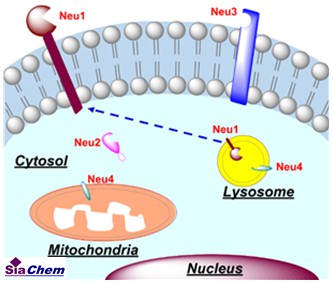

Sialidases (Neuraminidases, NAs) are glycosidases responsible for the removal of Sia residues (desialylation) from glycan portions of either glycoproteins or glycolipids [1,2]. In vertebrates, the sialidase family comprises four different forms in different subcellular localizations: the lysosomal sialidase Neu1, the soluble or cytosolic sialidase Neu2, the membrane-associated sialidase Neu3 and lysosomal or mitochondrial membrane associated sialidase Neu4 [1,2]. Each sialidase (Neu1, Neu2, Neu3 or Neu4) also has substrate specificity, suggesting different biological functions [3,4]. Neu1 is typically located in the lysosome, but also could relocate to plasma membrane upon stimulation, where it impacts receptor activation and signaling in a variety of cell types, including immune cells [5,6]. Various sialidases are also found in viral, bacterial, fungal, protozoan, avian, and mammalian species, which are often involved in infectious diseases, such as periodontitis, cystic fibrosis, pneumonia, vaginosis, and influenza [7]. Removing human Sias by pathogen sialidases contributes to uncontrolled signaling pathway in the pathological process [7,8].